Abstract
Background: Steroid-resistant (SR) intestinal acute graft versus host disease (aGVHD) is a devastating complication of allogeneic hematopoietic stem cell transplantation. Preliminary reports suggest that fecal microbiota transplantation (FMT) administered through a nasogastric tube or colonoscopy may be an effective treatment. We report the results of a single-arm pilot study (NCT 03214289) using FMT in capsules to treat SR or steroid dependent (SD) intestinal aGVHD.
Methods: The primary outcome was the occurrence of severe adverse events (SAEs) at 28 days post last FMT course. Secondary outcomes included GVHD response. Complete response (CR) was defined as resolution of gastrointestinal symptoms or reduction of steroid dose to 5 mg of prednisone. Partial response was defined as a decrease in severity of GVHD by at least one stage or a ≥40% reduction in steroid dose. Patients were eligible if they had SR or SD gut aGVHD without active infection or neutropenia. Per-protocol, participants received a course of 30 frozen capsules of fecal matter over two consecutive days. FMT courses could be repeated from the same or a different donor, at the treating physician's discretion. Capsules are produced from healthy unrelated donors who underwent vigorous screening. They are taken orally and are flavorless and odor-free. To characterize the impact of the FMT on the gut microbiota, stool samples of recipients were serially collected and underwent 16s rRNA sequencing.
Results: To date, we have enrolled 7 patients with intestinal aGVHD (6 SR, 1 SD) (Table). The median dose of methylprednisolone (MP) was 1 mg/kg (interquartile range [IQR] 0.8-1.3 mg/kg). FMT was administered at a median of 39 days (IQR 21-58 days) from aGVHD diagnosis. A total of 15 courses of FMT were given. Patients received a range of 1-3 FMT courses (median 2). The capsules were well tolerated. Patient #1 developed Enterococcus Faecium bacteremia 2 days following the second FMT. To track the source of bacteremia, we performed targeted metagenomic sequencing. The enterococcus strain from the blood culture was identified in the recipient's pre-FMT stool sample but not in the FMT inoculum (i.e., capsule), confirming that the bacteremia was not an FMT complication. Similarly, patient #6 developed Pseudomonas aeruginosa bacteremia 3 days after the 2nd FMT. 16s rRNA sequencing of the donor capsule failed to demonstrate Pseudomonas taxa. No other SAEs suspected to be related to the FMT were observed. Two patients achieved a CR with complete resolution of GVHD symptoms. Patient #6 had a partial improvement following the 1st FMT, with a reduction of MP from 2 mg/kg to 1.3 mg/kg. Three days after the 2nd FMT, she developed fatal pseudomonas bacteremia, not related to the FMT as detailed above. At last follow-up (median 61 days, IQR 40-99), 3/7 patients were alive. Three patients died from consequences of active GVHD, while one patients who responded to FMT and was free of GVHD, succumbed to an invasive Aspergillus infection of the brain.
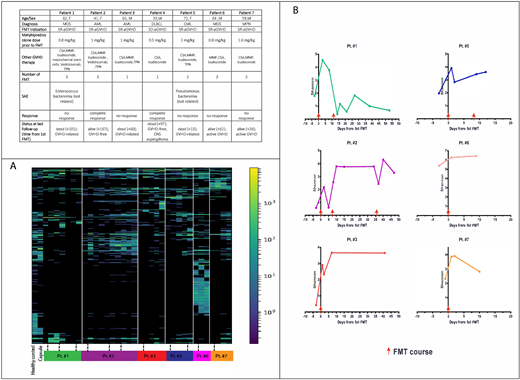
16s rRNA sequencing of stool samples revealed bacterial domination (i.e., occupation of at least 40% of the microbiota by a single predominating taxon) of Escherichia(E) coli in four patients before FMT, with a major reduction following therapy. FMT was associated with the introduction of new bacteria and an increase in bacterial diversity in the recipient's stool (Figure).
Conclusions: We demonstrate for the first time the utility of fecal microbiota transplantation in orally administered capsules for the treatment of severe intestinal acute GVHD. The capsules were well tolerated and safe. Metagenomic sequencing proved that a bacterial infection following FMT was not related to the procedure. Sequencing of the stool sample revealed bacterial domination with E.coli in 4/7 patients prior to the first FMT. Following FMT, bacterial diversity increased. Finally, 2/7 patients attained a complete response following therapy, suggesting a potential role of FMT in patient management.
Figure. (A) Heatmap of operational taxonomics units (OTU). Each column marks a sequenced stool sample at a specific time point and rows individual taxas. The color code indicates relative abundance. Dotted lines represent an FMT course. Before FMT all patients, aside from patient #6, had markedly reduced diversity, with enrichment of OTUs following treatment. (B). Change of bacterial diversity, measured by the Shanon diversity index before and after FMTs.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal